In the aftermath of WEGO Medical’s founding, the landscape was rife with challenges – a singular product, incomplete specifications, and a noticeable lack of refinement in quality. The marketplace, saturated with fierce competition, presented a formidable situation for the enterprise. Undeterred by the hurdles, WEGO Medical recognized that adhering exclusively to existing products would amount to self-imposed limitations, obstructing both survival and growth.
Faced with this reality, WEGO Medical embraced a visionary approach, acknowledging the imperative of technological progress. The strategic shift involved not only diversifying the product range but also incorporating cutting-edge technologies. This multifaceted strategy aimed to create a robust system encompassing the production of one generation, the accumulation of reserves, the research and development of another generation, and the visionary conceptualization of yet another generation.
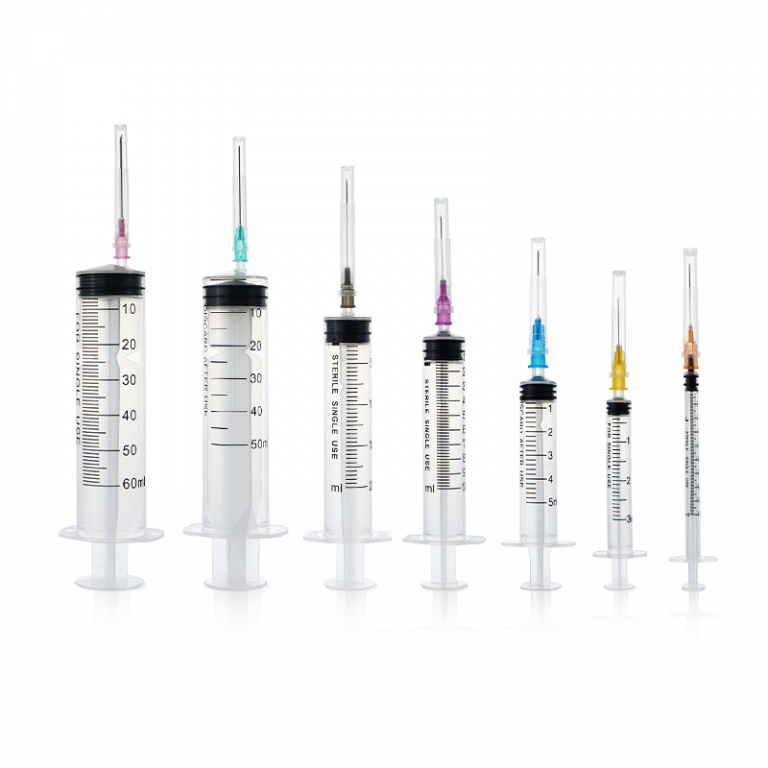
In April 1989, WEGO Medical embarked on an ambitious initiative to enhance its operational capabilities and bolster product competitiveness. Collaborating with esteemed institutions such as the Shandong Provincial Medical Equipment Research Institute and Qingdao Ocean University, the company invested millions in researching and developing PVC materials and disposable syringes.
By July of the same year, WEGO Medical achieved a significant milestone by obtaining the first nationwide industrial product production license for disposable infusion sets, bestowed by the National Medical Products Administration. This recognition signified the company’s commitment to quality and compliance with national standards, setting the stage for a robust and competitive product portfolio.
In October 1989, WEGO Medical underwent a transformative evolution, rebranding itself as the Shandong Provincial Weihai Medical Polymeric Products General Factory. The restructuring included the expansion of the industrial scale, with the establishment of three specialized divisions for infusion sets, polymer materials, and syringes. This strategic move allowed WEGO Medical to diversify its product range, introducing a variety of medical solutions to meet evolving market demands.
Undeterred by challenges, the company continued to expand its footprint. In May 1990, a fourth division was established for the research and development of pediatric scalp needles, steel needles, and other innovative products. Recognizing the paramount importance of quality assurance, WEGO Medical, early in its developmental journey, instilled a robust quality consciousness. In July 1990, the company invested over 34,000 yuan to establish a laboratory dedicated to product testing. By the end of that year, WEGO Medical’s commitment to quality was acknowledged through a standardization assessment by the Shandong Provincial Bureau of Quality and Metrology.
The year 1990 marked a turning point as national authorities, including leaders from the National Science and Technology Commission and the Ministry of Health, visited the company. Their commendation of WEGO Medical’s commitment to high standards and formalized business practices led to the collaboration with the Shandong Provincial Medical Equipment Research Institute on a high-tech project under the National Torch Program. This endeavor aimed at developing highly efficient water-absorbing resin products, making it the company’s inaugural National Torch Program project.
Simultaneously, WEGO Medical expanded its presence by establishing offices in multiple cities, including Yanwei, Qingdao, Dalian, and Benxi. This strategic approach allowed WEGO Medical’s products to transcend Shandong, gradually achieving nationwide coverage. The company’s commitment to quality, innovation, and national collaborations laid the groundwork for its ascent as a prominent player in the medical industry.